Another Review Confirms the Problem — But Integration Is Still Missing
- Healing_ Passion
- 7 days ago
- 3 min read
Why mitochondrial dysfunction links aging, insulin resistance, and inflammation — and why medicine still treats them separately.
A recent review published in Metabolites (2025) by Hao et al., “Relationship of Ageing to Insulin Resistance and Atherosclerosis”, delivers a clear and important message:
Aging, insulin resistance, chronic inflammation, and atherosclerosis are not separate diseases — they are biologically connected through mitochondrial dysfunction.
This is not a radical claim. But the way the review assembles the evidence makes something undeniable: mitochondrial failure sits upstream, while insulin resistance (IR), chronic systemic inflammation (CSI), and vascular dysfunction appear downstream as linked consequences.
And yet — despite this convergence — our medical and research systems still treat these conditions as if they were unrelated.
What the Metabolites review clearly establishes
The review by Hao and colleagues synthesizes evidence showing that:
Aging is accompanied by declining mitochondrial efficiency and impaired mitophagy
Mitochondrial dysfunction increases reactive oxygen species (ROS) and redox imbalance
These changes impair insulin signaling, promote hyperinsulinemia, and drive endothelial dysfunction
Chronic low-grade inflammation (“inflammaging”) emerges through persistent immune activation
Insulin resistance and atherosclerosis amplify one another in a self-reinforcing cycle
Crucially, the review frames mitochondrial dysfunction as an upstream driver, not merely a downstream casualty.
This alone challenges glucose-centric and cholesterol-centric thinking.
But it also raises an uncomfortable question:
If we already know these processes are connected, why do we still treat them as separate problems?
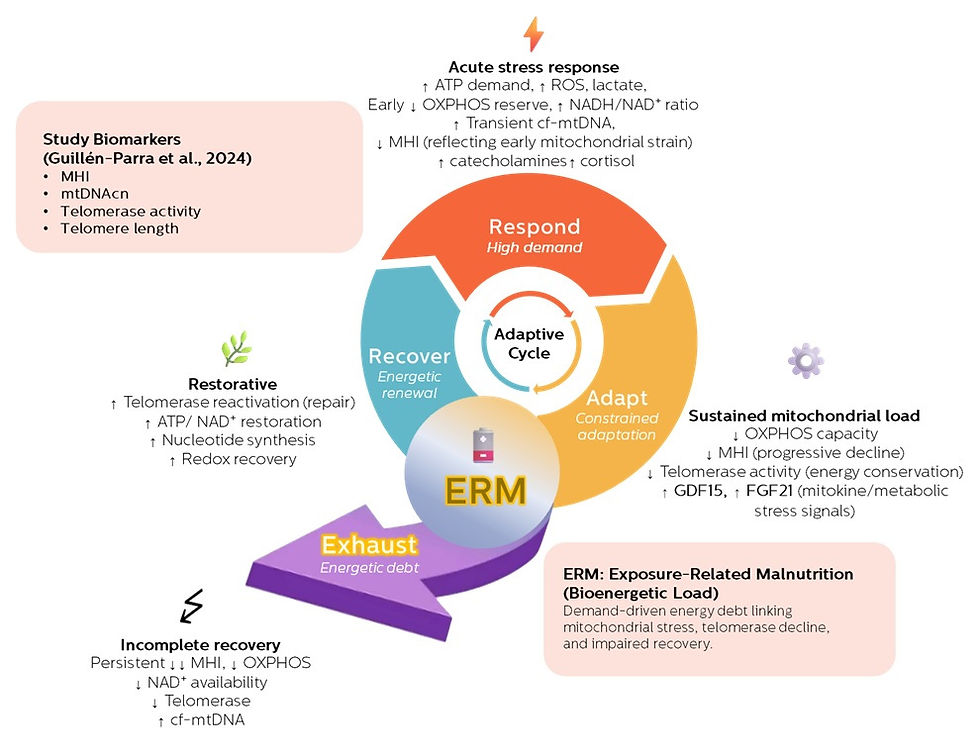
Where the Exposure-Related Malnutrition (ERM) framework adds what’s missing
This is where the ERM framework becomes relevant.
ERM does not contradict the review — it extends it by offering a continuous, energetically grounded explanation of how these conditions emerge over time.
ERM reframes the story as a metabolic continuum:
Chronic exposures
Nutritional mismatch, psychosocial stress, inflammation, toxins, and sleep disruption
Metabolic stress adaptation
The body reallocates energy toward survival
Mitochondrial constraint
ATP production, redox balance, and recovery capacity become limited
Downstream expressions
ROS accumulation
Insulin resistance as an adaptive brake
Chronic sterile inflammation
Progressive tissue and vascular dysfunction
In this model, insulin resistance and inflammation are not primary diseases — they are signals that energetic resolution has failed.
The Metabolites review describes the mechanisms.ERM provides the directionality and continuity.
Why fragmentation persists — despite reviews like this one
Even with reviews as integrative as Hao et al., three structural problems remain.
1. Medicine is organized around endpoints, not trajectories
Diabetes focuses on glucose
Cardiology focuses on plaques
Immunology focuses on inflammation
Aging research focuses on senescence
Each field studies a snapshot, not the adaptive journey.
ERM focuses on the path from exposure to failure, not the endpoint alone.
2. Stress adaptation is discussed without energetic accounting
Concepts like resilience, hormesis, and allostasis are widely used — but rarely measured in energetic terms.
ERM makes a simple but disruptive claim:
Adaptation has a metabolic cost. If recovery is incomplete, energetic debt accumulates.
The review implies this.ERM makes it explicit.
3. Mitochondria are acknowledged — but not treated as system governors
In most models, mitochondria are:
Damaged by inflammation
Worn down by aging
Secondary to disease
ERM places mitochondria where the review’s evidence already points:
as the limiting infrastructure for resolution, repair, and resilience.
Without restoring mitochondrial capacity, suppressing downstream signals cannot fully restore health.
Why this matters clinically
When conditions are treated in isolation:
Glucose improves, but fatigue remains
Inflammation markers fall, but recovery doesn’t return
Lipids normalize, but resilience continues to decline
ERM reframes the clinical question:
Where is this person along the exposure → adaptation → constraint → failure continuum?
That question changes:
What we measure
When we intervene
What “improvement” truly means
A hopeful conclusion
The review by Hao et al. (Metabolites, 2025) strengthens an essential point:aging-related metabolic disease follows a coherent biological logic.
ERM adds what is still missing:
A continuous trajectory
An energetic accounting of adaptation
A unifying explanation for why IR, CSI, and dysfunction cluster together
And a framework for earlier, more meaningful intervention
The obstacle is no longer a lack of data.
It is a lack of integration.
And integration is exactly where the next phase of metabolic and aging medicine must go.
Hao, X., et al. (2025). Relationship of ageing to insulin resistance and atherosclerosis. Metabolites, 15(6), 613. https://doi.org/10.3390/metabo15060613




Comments