Our New Publication: Human Evidence That Chronic Stress Ages Us Through Bioenergetic Debt
- Healing_ Passion
- 5 days ago
- 3 min read
We’re pleased to share that our review has just been published in Biogerontology.
In this paper, we synthesize human evidence showing that chronic psychological stress accelerates biological aging not primarily through irreversible DNA damage—but through progressive failure of energetic recovery, a process we describe as bioenergetic debt
.
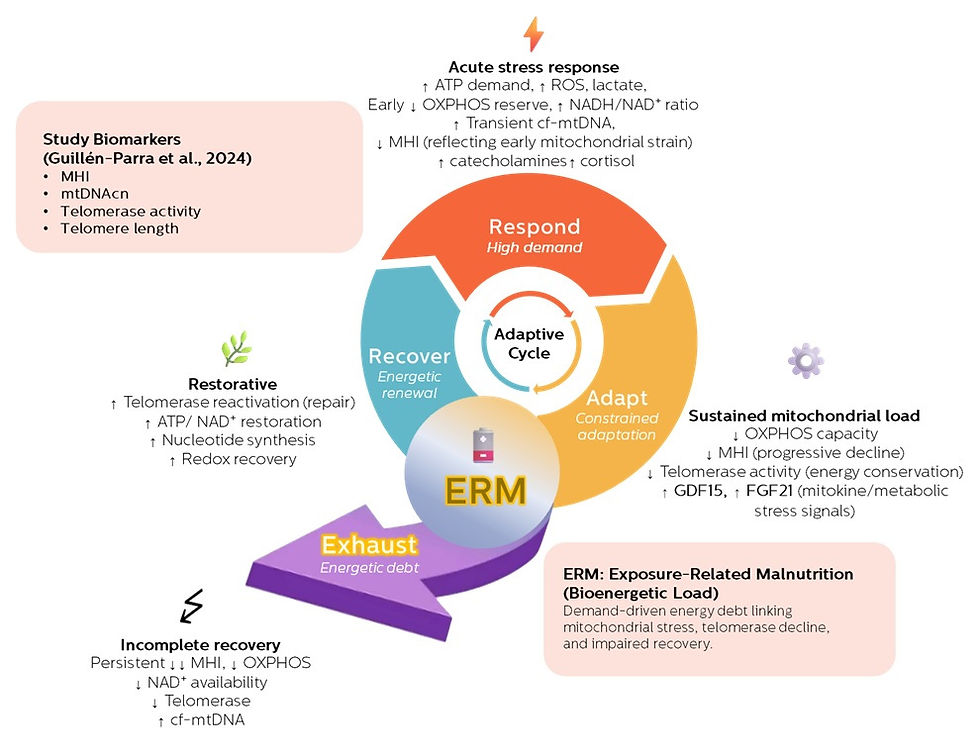
This work brings together longitudinal human data, mitochondrial biology, and telomere science, and provides direct empirical support for the Exposure-Related Malnutrition (ERM) framework we have been developing—positioning aging and stress-related disease as consequences of unresolved energetic strain.
Stress Is Not the Problem—Failure to Recover Is
A central message of our review is that stress itself is not inherently harmful. Biology has long recognized the benefits of physiological hormesis: mild stressors, when followed by adequate recovery, strengthen mitochondrial function, redox balance, and cellular maintenance.
The problem arises when stress becomes chronic, repetitive, and poorly resolved.
Modern life exposes many individuals to sustained psychosocial stress without sufficient time, substrates, or metabolic capacity for full recovery. Over time, this mismatch accumulates—not as an immediate disease, but as a growing energetic shortfall.
Human Evidence for a Mitochondria–Telomere Axis
Our review is anchored in longitudinal human data from the Guillén-Parra caregiver cohort, a well-characterized model of chronic psychosocial stress. Importantly, these participants were otherwise healthy, non-smoking, and free of major metabolic or psychiatric disease.
What the data reveal is striking:
Lower baseline mitochondrial energetic capacity predicted greater declines in telomerase activity over time
Telomerase activity declined before telomere length changed
Telomere shortening, when present, followed telomerase suppression rather than stress exposure directly
This pattern provides rare in vivo human evidence that mitochondrial energetics sit upstream of telomere maintenance under chronic stress.
Telomerase activity is not merely a genetic program—it is an energy-dependent process, requiring ATP, NAD⁺, redox balance, and intact mitochondrial signaling. When mitochondrial throughput is constrained, telomerase becomes one of the earliest systems to fail.
How ERM Explains These Findings
The ERM framework offers a mechanistic explanation for why these patterns emerge.
ERM conceptualizes chronic stress as a persistent metabolic demand that repeatedly activates catabolic stress responses. If recovery phases are truncated or energetically under-resourced, the organism never fully restores mitochondrial function, redox balance, protein synthesis, and genome maintenance.
Over time, this leads to:
Reduced mitochondrial efficiency (lower energetic throughput per organelle)
Impaired NAD⁺ regeneration and redox recovery
Substrate misallocation toward storage rather than repair
Suppression of energetically costly maintenance processes, including telomerase activity
This is not classic malnutrition. Calories may be abundant.
What is lacking is functional energy availability for repair.
That state—adequate intake but inadequate energetic resolution—is what we define as Exposure-Related Malnutrition.
Aging as a Failure of Resolution, Not Just Damage
One of the key implications of our review is a reframing of aging itself.
Rather than viewing aging as the passive accumulation of molecular damage, our synthesis aligns with contemporary geroscience perspectives that define aging as a progressive failure of adaptive resolution—a declining ability to fully recover after stress.
Within this view:
Telomerase decline is an early, energy-sensitive marker of vulnerability
Telomere shortening is a delayed structural consequence
Aging trajectories are shaped by recovery capacity, not stress exposure alone
This perspective integrates naturally with concepts such as homeodynamic space, catabolic–anabolic cycling hormesis, and resilience biology.
Why This Matters for Prevention and Care
Because bioenergetic debt develops before irreversible damage, it represents a potentially modifiable stage.
Our review highlights the importance of strategies that restore recovery capacity, including:
Circadian alignment and sleep regularity
Structured feeding–fasting rhythms
Adequate protein and micronutrient sufficiency
Exercise that respects recovery, not just intensity
Interventions that improve mitochondrial efficiency rather than simply suppressing inflammation
We also emphasize the need for dynamic biomarkers of energetic strain and recovery, such as mitochondrial health indices, NAD⁺ status, circulating cell-free mitochondrial DNA, and mitokines like GDF15—markers that reflect energetic load before overt disease appears.
A Step Toward an Integrated Biology of Stress and Aging
This publication represents an important step in connecting stress biology, mitochondrial energetics, telomere maintenance, and aging into a single, energy-dependent framework.
From the ERM perspective, early aging is not an inevitable decline—it is often deferred repair. And where recovery can be restored, resilience may still be rebuilt.
Reference
Tippairote T, Hoonkaew P, Suksawang A, Tippairote P. Chronic stress and the mitochondria–telomere axis: human evidence for a bioenergetic-debt model of early aging. Biogerontology (2026). https://doi.org/10.1007/s10522-025-10377-x
Read the review here: https://rdcu.be/eXLbo




Comments