Why Stress Doesn’t Break Us—But Failed Recovery Does
- Healing_ Passion
- 2 days ago
- 3 min read
New evidence places mitochondria at the center of aging, pain, and metabolic dysfunction
For decades, stress has been blamed as the primary driver of aging, chronic disease, and burnout. Too much cortisol. Too much inflammation. Too much pressure on the nervous system.
But a growing body of research is telling a different story.
Stress, it turns out, is not the real villain. Failed recovery is.
A newly published study in Nature adds powerful biological evidence to this shift in thinking—showing that mitochondria are not just damaged by stress, but actively determine whether stress adaptation resolves or collapses into dysfunction, pain, and accelerated aging.
A surprising discovery: cells share mitochondria to survive stress
In this study, researchers examined what happens to sensory neurons under stress, injury, and metabolic disease. What they found was remarkable.
When neurons became hyperactive or stressed, neighboring support cells—called satellite glial cells—actively transferred mitochondria directly into neurons. This wasn’t random.
It was:
Targeted
Activity-dependent
Local and purposeful
In effect, cells were sharing their energy-producing infrastructure to help stressed neurons cope.
As long as this mitochondrial support system worked, neurons remained stable—even under ongoing stress.
But when mitochondrial transfer was disrupted—by diabetes, chemotherapy, or damage to the cellular structures that enable this exchange—neurons quickly developed:
Energy instability
Oxidative stress
Abnormal electrical firing
Axonal damage
And ultimately, chronic pain
Crucially, these failures occurred before neurons died and even when short-term ATP levels appeared “normal.”
This tells us something important:
The problem isn’t lack of energy supply. It’s lack of mitochondrial capacity to support recovery.
What this means: mitochondria are not passengers—they are the bottleneck
This study confirms a core insight that has been emerging across aging, metabolism, neuroscience, and stress biology:
Mitochondria are the rate-limiting infrastructure of recovery.
Stress responses—like inflammation, vigilance, and catabolism—can continue even when mitochondria are under strain. They are designed for survival.
But recovery is different.
Recovery requires:
High-quality ATP
Redox balance
Protein synthesis
Cellular repair
Renewal and rebuilding
When mitochondria can no longer support these processes efficiently, the body doesn’t “shut down.”It adapts.
And over time, that adaptation becomes costly.
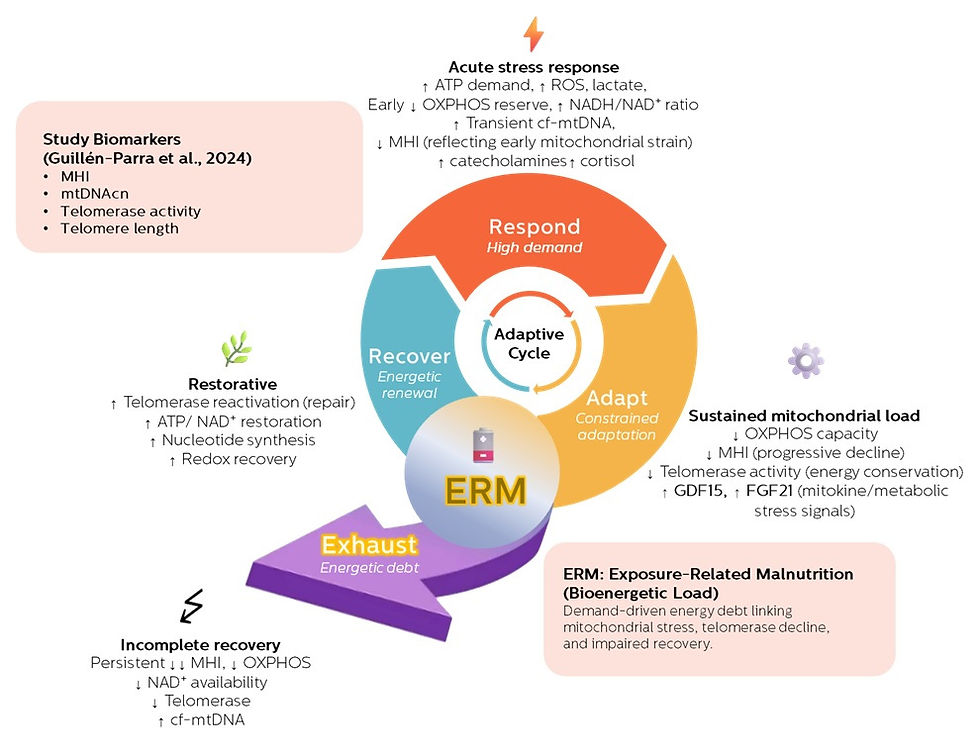
How this aligns with the ERM framework
This is where the findings align directly with the Exposure-Related Malnutrition (ERM) framework.
ERM does not describe starvation or calorie deficiency. It describes a state where:
Energetic demand chronically exceeds the body’s capacity for ATP-dependent recovery—despite adequate intake.
In ERM:
Stress adaptation continues
Survival is preserved
But recovery is repeatedly postponed
The result is a gradual accumulation of bioenergetic debt.
This study shows that even at the cellular level, the body tries to compensate—by redistributing mitochondria between cells. But when that adaptive support fails, dysfunction follows.
Pain, insulin resistance, inflammation, fatigue, and cognitive symptoms are not primary defects.
They are signals that recovery has become biologically unaffordable.
Rethinking pain, aging, and metabolic disease
One of the most striking implications of this research is how it reframes chronic pain.
Pain is often treated as:
A nerve signaling problem
An inflammatory disorder
A psychological condition
But this study shows pain emerging as a downstream consequence of mitochondrial failure to support recovery.
The same logic likely applies more broadly:
Insulin resistance may act as a protective gate to limit further energetic overload
Chronic inflammation reflects immune activity under constrained repair capacity
Muscle loss and fatigue arise when re-anabolism is persistently suppressed
Aging itself may reflect cumulative failures of mitochondrial-supported resolution
From this perspective, aging is not simply damage accumulation—it is adaptation without energetic resolution.
What about future mitochondrial interventions?
This research also points toward a promising, but nuanced, future.
If mitochondria determine whether stress resolves or becomes pathological, then interventions that restore mitochondrial processing capacity and recovery efficiency may change the trajectory of aging and metabolic disease.
This does not mean quick fixes or “mitochondria boosters.”
It suggests the need for:
Supporting mitochondrial throughput, not just ATP quantity
Restoring redox balance and metabolic flexibility
Reducing chronic energetic congestion
Timing interventions to support recovery, not just performance
Lifestyle strategies, nutrition, circadian alignment, physical activity, and carefully targeted metabolic therapies may all play a role—but only if they reduce bioenergetic debt rather than increase demand.
The takeaway
This study reinforces a profound but hopeful message:
You’re not broken.You’re exhausted at the cellular level.
Stress is inevitable.
But recovery failure is modifiable.
By placing mitochondria at the center of stress adaptation, aging, pain, and metabolic health, this research strengthens a new paradigm—one where resilience is not about pushing harder, but about restoring the energy required to heal.
And that changes everything.
Xu, J., Li, Y., Novak, C. et al. Mitochondrial transfer from glia to neurons protects against peripheral neuropathy. Nature (2026). https://doi.org/10.1038/s41586-025-09896-x




Comments